Abstract
Objective
To review the efficacy and safety of specific herbal medications that have been used traditionally to treat common conditions in women.
Data Sources
Current literature, with emphasis on more rigorously controlled studies.
Data Synthesis
Herbal medicines have long been used in traditional healing systems to treat conditions of particular interest to women, such as premenstrual syndrome (PMS) and menopausal symptoms. For a select number of phytomedicines, including evening primrose oil, black cohosh root extract, dong quai, and chaste tree berry, scientific investigation is elucidating the pharmacologically active constituents, mechanism of action, and clinical value.
Conclusion
Based on the available evidence, evening primrose oil and chaste tree berry may be reasonable treatment alternatives for some patients with PMS. Dong quai may have some efficacy for PMS when used in traditional Chinese multiple-herb formulas. For relief of menopausal symptoms, black cohosh root extract and dong quai have good safety profiles, but only black cohosh has demonstrated efficacy for this indication. Safety data, especially during pregnancy and lactation, are still largely lacking for many herbal medications, and recommendations for usage and dosage vary. Pharmacists who wish to recommend herbal products for women's health conditions need to evaluate the scientific literature in order to form their own opinions about appropriate use and safety.
Introduction
Herbalism, the study and use of plants for medicinal and preventive purposes, is an art that predates written history.[1] Some version of an herbal tradition has been found in all the great cultures of the world, from the East (China, Japan, India), through the aboriginal cultures of Australia, Africa, and the Americas, to the foundational cultures of the West (Egypt, Greece, Rome, and Europe). In many of these cultures, women were and are prominent practitioners of herbal medicine, especially as it relates to women's medicine. In the Egyptian pantheon, the goddess Isis was identified as the source of healing herbs. In the 11th century, Hildegard of Bingen, a Christian mystic and healer, wrote extensively on the healing properties of herbs. Skilled lay herbalists, frequently working as midwives, were taken for witches during the Inquisition. To some degree, it was their knowledge of arcane herbal lore, such as the famous "flying ointments" (likely extracts of high-alkaloid herbs) that made them suspect. In fact, women herbalists were the repository of much useful herbal lore -- it was the Old Woman of Shropshire who taught Withering how to use foxglove. Many shamanic traditions honor women's knowledge of herbs for sacred and mundane uses.[1]
Because women often cared for the physical complaints of other women, and, at least in European cultures from the Middle Ages on, were excluded from sanctioned medical practice, it is not surprising that the herbal pharmacopoeia is especially rich in therapeutics for women. In this century, many of these traditional remedies have started to be studied scientifically and used in common practice.
Modern herbal practice today still provides women with many useful herbal remedies for conditions of particular interest to them, especially those associated with the menstrual cycle, pregnancy, and menopause. This article examines the scientific evidence for evening primrose oil, chaste tree berry, dong quai, and black cohosh. Some cautionary evidence on the use of herbs in pregnancy and lactation is presented, and several less well-known but promising herbs traditionally used to treat women are described. The objective of this article is to familiarize pharmacists with some of the more rigorous studies that have evaluated the use of herbs in women's health, to aid them in forming opinions about the appropriateness of use.
Evening Primrose Oil
Evening primrose, Oenothera biennis L., is a North American wildflower that has escaped cultivation and is now widely distributed in fields or along roadsides.[2] Named so because its flower opens in the evening, the evening primrose is in fact not a true primrose. Traditionally, the plant was valued as a food, and its roots and seeds were eaten.[3] Medicinal use of evening primrose has a long history among Native Americans, and use of the plant was transferred to Europe by colonial settlers.
Modern interest has centered on the oil expressed from the plant's small dark seeds. The seeds produce a fixed oil rich in essential fatty acids: approximately 65% linoleic acid and 8% to 10% gamma-linolenic acid.[4,5] These constituents are critical precursors in the manufacture of prostaglandin E1, one of the anti-inflammatory prostaglandins.[6]
A number of studies have evaluated the efficacy of evening primrose oil (EPO) in the treatment of premenstrual syndrome (PMS). The rationale for this use is that women with PMS have an abnormal profile of essential fatty acids, which may be normalized by supplementation with EPO.[6-9] Brush et al.[7] reported that women with PMS have high levels of n6 essential fatty acids but low levels of all metabolites of linoleic acid, including arachidonic acid. These investigators reported that gamma-linolenic acid levels were below detectable levels in PMS patients, and postulated that PMS is associated with a defect in the conversion of linoleic acid to gamma-linolenic acid. Abnormal levels of essential fatty acids also have been observed in women with benign breast disorders.[8]
Clinical Studies
In an open label study, Brush[10] evaluated the efficacy of EPO for PMS symptoms in 68 women. The women received EPO (1 to 2 grams per day) from 3 days before the usual onset of their PMS symptoms until the onset of menses. Based on a patient self-report scale, 41 women (61%) had complete relief of their symptoms and 16 (23%) had partial relief after at least 3 months of treatment. The most pronounced symptom relief was for mastalgia (breast pain).
Many additional trials, such as those conducted by Larson et al.[11] and Ylikorkala et al.,[12] have reported modest benefits of EPO for PMS symptoms. However, these favorable effects were not replicated in a double-blind, placebo-controlled crossover trial of 38 women with PMS.[13] This study, which used a daily EPO dose of eight capsules (presumably 4 grams), failed to demonstrate statistically or clinically significant results.[13] Furthermore, an attempted meta-analysis of the effects of EPO on general PMS symptoms also did not demonstrate conclusive efficacy.[14] The majority of trials cited suffered from methodologic flaws that made meta-analysis difficult, such as an open study design with no placebo control and a placebo-controlled, parallel-group study without a defined randomization scheme.[11,15,16]
Although clinical studies have not shown a clear benefit of EPO for PMS, more pronounced improvement has been demonstrated for relief of mastalgia. A double-blind, placebo-controlled study compared the effects of danazol, bromocriptine, EPO, progestins, and placebo in 291 women with mastalgia.[17] The experimental agents were tried sequentially, and the patients subjectively rated their relief by recording their assessment of pain on a visual analogue scale supplemented by a pain diary. EPO (3 grams per day) was effective for cyclical mastalgia in 45% of patients, with a relapse rate of 21% after the first course of treatment. Effectiveness was defined as either a Grade I response (no residual pain) or a Grade II response (some residual pain that the patient described as easily bearable).
Only 2% of the EPO-treated patients reported side effects (mild bloating with vague nausea).[17] Compared with danazol, EPO was less effective (70% versus 45%), but had fewer side effects (22% versus 2%). EPO had similar efficacy to bromocriptine (47% versus 45%), again with fewer side effects (2% versus 33%). Effectiveness rates for all therapies were lower in patients with noncyclical mastalgia, with danazol showing a 31% response rate; EPO, 27%; bromocriptine, 20%; and progesterone, 9%.
A much lower response rate was recently reported in a clinical survey conducted at a hospital-based mastalgia clinic in Australia, which recorded observations on 170 patients for 3 years.[18] Response rates of only 26% were reported for mastalgia patients receiving EPO, while low-dose danazol achieved an effect in 67% of the patients.
Despite the mixed results of these studies, many clinicians recommend EPO as a first-line treatment for cyclic mastalgia.[8,19-25] For example, in a recent survey 13% to 30% of British surgeons recommended EPO for this use.[24] Patients with severe PMS symptoms, in another recent survey,[26] also rated EPO as one of the most effective treatments they had used.
Considerations for Patient use
Given its good safety profile, EPO can reasonably be tried for PMS at a dose of 2 to 4 grams (standardized to 9% gamma-linolenic acid), especially if mastalgia is present. EPO also may confer benefit for other symptoms associated with PMS, such as irritable bowel syndrome.[27] A critical analysis is under way to further examine questions of efficacy at the Cochrane Collaboration, which is producing a compendium of systematic evidence-based reviews.[28]
Given its reputed usefulness in PMS, EPO has also been tried for management of menopausal symptoms, but its use for this indication has much less data to recommend it. A double-blind, placebo-controlled trial of 56 menopausal women failed to demonstrate a statistically significant effect of EPO (2 grams/day) on hot flushes.[29] Although a small, statistically significant improvement in the number of nighttime flushing episodes (P < .05) was recorded, the study did not report whether the patients considered this improvement to be significant.

Figure 1. Evening primrose. Adapted from: Herbal Plants: History and Uses. London: Studio Editions Ltd. Copyright 1991
Chaste Tree Berry
The dried ripe fruit of the chaste tree, Vitex agnus-castus L., has been used as a medicine since ancient Greece and was cited by both Hippocrates and Discorides for its effects on female reproduction.[30] Medieval use focused on the small pepper-like berry's ability to decrease sexual desire in men (specifically, monks), hence the common name chaste tree or monk's pepper.[31] Although the active principles of Vitex have not been conclusively determined, modern analysis has isolated two iridoid glycosides[lparentop]agnuside (0.6%) and aucubin (0.3%),[32] flavonoids (0.45 to 0.97% in the fruit),[32,33] and essential oils.[33] When products are standardized, they are usually standardized to one of the two glycosides mentioned above.
Several hormones have been isolated from the leaves and flowers of Vitex, including progesterone, hydroxyprogesterone, testosterone, and androstenedione.[5,31,33,34] However, these hormones are not likely of clinical significance, because they are found in very small amounts and distributed mainly in the leaves and flowers, not in the main medicinal constituent, the fruit.[33]
Chaste tree berry extract is believed to exert its clinical action through its dopaminergic effects on the anterior pituitary. Animal and human studies have shown that extracts of chaste tree bind to dopamine2 receptors in the anterior pituitary and decrease both basal- and thyrotropin-releasing-hormone-stimulated secretion of prolactin.[35-38] This decrease in prolactin leads to increased progesterone production in the luteal phase of the menstrual cycle, which reduces symptoms of PMS.[39] Consistent with this theory, PMS sufferers have significantly higher rates of prolactin throughout their cycles, especially in the second and third weeks.[40]\ Vitex has been postulated to correct hyperprolactinemia, thus allowing normal corpus luteum development and preventing PMS.[41]
Clinical Studies
Three large uncontrolled drug monitoring studies reported the effects of Vitex agnus-castus extract (VACE; 50% to 70% V/V alcoholic-aqueous extract) on a variety of menstrual abnormalities, including PMS and irregular menses.[42-44] The pooled results from more than 4,500 patients showed 29% to 33% of the women reporting, via self-rating tools, complete relief from their symptoms and 52% to 57% with marked improvement. Relief was typically seen in responders after three menstrual cycles of treatment.[42-44] The dosage was 40 to 42 drops of a liquid VACE (effective daily dose 30 to 40 mg of plant extract). Less than 2% of patients reported adverse effects; these were minor and included gastrointestinal upset, itching, mild rash, headaches, and increased menstrual flow.
The ability of VACE to normalize luteal phase defects was tested in a randomized double-blind, placebo-controlled trial of 52 women with latent hyperprolactinemia and abnormal thyrotropin-releasing hormone stimulation tests.[45] The women received either 20 mg VACE per day (lower than the standard dose) or placebo for 3 months. In the VACE-treated women, the luteal phase lengthened by 5 days with normal mid-luteal progesterone levels. Patients with symptomatic PMS reported relief of symptoms. Of note, 2 of the 17 women in the VACE group became pregnant. These findings are consistent with a previous uncontrolled study by Propping et al.,[46] in which VACE was tested in 45 women with corpus luteum deficiency. At a dose of 40 drops per day (presumably 30 to 40 mg/day of crude plant extract), 25 of the patients normalized their serum progesterone levels after 3 months of therapy and 7 women, who had previously had difficulty conceiving, became pregnant.
In another study, 175 women with premenstrual tension syndrome were evaluated for three consecutive menstrual cycles during treatment with a proprietary chaste tree berry extract product (3.5 to 4.2 mg daily throughout the menstrual cycle) or pyridoxine (100 mg twice daily for the second half of the menstrual cycle).[47] Placebo capsules were used to make the dosage schedules of the two medications equivalent so that the participants were blinded as to which medication they were taking, but neither treatment was compared directly with placebo.
At the end of the treatment period, 126 subjects were available for analysis: 61 in the Vitex group and 66 in the pyridoxine group.[47] Outcomes were recorded using the Premenstrual Tension Scale and the Clinical Global Impression Scale. The 20 women who terminated treatment prematurely were not included in the efficacy analysis; thus an intent-to-treat analysis was not done. Therapeutic outcomes were very similar in the Vitex- and pyroxidine-treated groups. Statistically significant improvements relative to baseline were reported in Premenstrual Tension Scale scores for both groups (P = .03 for Vitex). Clinical Global Impression scores were also improved, with 77.1% of patients reporting improvement in symptoms. Efficacy, as determined by the investigators using the same scale, was also positive, and 24.6% of the physicians rated the Vitex-treated group as excellent. At the end of the trial, 36% of the Vitex users reported that they were symptom free. Only minor adverse effects (mild nausea and mild rashes) were reported.
Concern about the potential for VACE to cause ovarian hyperstimulation was raised in a published case report,[48] in which a woman who underwent unstimulated in vitro fertilization developed mild ovarian hyperstimulation (demonstrated by ultrasound), apparently related to a chaste tree berry medication. The condition resolved once the herb was withdrawn. Because the dose and preparation of the product she was taking were not reported, extrapolation of this finding to other patients is difficult. Nonetheless, the authors were concerned about the potential for VACE to cause ovarian hyperstimulation syndrome, which can be life-threatening.
Considerations for Patient use
Concomitant use of VACE with oral contraceptives is not recommended, because of a possible decrease in contraceptive efficacy via the effects of VACE on prolactin. For the same reason, VACE is best avoided in pregnancy.[49]
Based on the available evidence of efficacy and safety, chaste tree berry extract is a reasonable phytomedicine to recommend for the treatment of PMS and abnormal menses, especially if a progesterone deficiency is suspected. No direct comparison studies have been done between VACE and progesterone, a standard treatment for PMS. VACE also may have some benefit as a fertility agent.[50]
For clinicians who wish to use this phytomedicine, dosage decisions are difficult because of the imprecision in reported dosages in the published literature. Current dosage recommendations for water-alcohol extracts are preparations that provide an average daily dose equivalent to 20 to 40 mg of fresh berries. Preparations have also been standardized to contain 0.5% agnuside. For this type of product, the recommended dosage is 175 to 225 mg per day.[50]

Figure 2. Chaste tree berry. Adapted from: Herbal Plants: History and Uses. London: Studio Editions Ltd. Copyright 1991
Dong Quai
The root of Angelica sinensis (Oliv.) Diels, known as dong quai in traditional Chinese medicine, is popular among women as a tonic. It is often referred to as the "female ginseng," and may be recommended for a wide variety of conditions. Classified as a blood tonic in traditional Chinese medicine, this herb is highly respected as a tonifier (a product designed to restore normal function over time) and as a decongestant in Body organs. Only licorice is found more often in Chinese formulas.[51-55]
In women's medicine, dong quai may be recommended for dysmenorrhea (a primary indication), irregular menstruation, abdominal pain or constipation, anemia, and as a supportive herb for menopausal complaints. In traditional Chinese medicine, herbs are almost never used as single agents, but are combined into complex formulas. In the West, dong quai is typically used alone or in combination with other herbs from traditional European sources.[56]
A scientific rationale for the observed improvements in anemia, PMS, irregular menses, and other menstrual complaints can be inferred by looking at the active constituents of dong quai. Significant amounts of vitamin B12 (0.25 to 0.4 µg/100 grams dried root), folic acid, and nicotinic acid are found in the root, thus providing a partial explanation for the beneficial effects on anemia. Ferulic acid (a phenolic organic acid found in the root) has been reported to have anti-inflammatory and analgesic effects. The action of dong quai on the uterus is complex. Water-soluble fractions stimulate the uterus, while alcohol-soluble constituents promote relaxation, probably via the action of ligustilide, a major constituent of the essential oil.[53] Direct estrogenic activity of the root extract has not been observed, but some sources, citing mainly animal models, report a progestational effect, which would be helpful in many cases of PMS.[51-53,57]
Clinical studies
In the West, dong quai has gained an identity as the "menopause herb" -- a reputation that the available scientific evidence does not support. The estrogenic effects of dong quai in 71 postmenopausal women were tested in a recent double-blind, placebo-controlled trial.[58] All women had been postmenopausal for at least 6 months, and had significant menopausal symptoms with normal Pap tests and mammograms. Participants were evaluated for endometrial thickness (by transvaginal ultrasound), vaginal cytology, serum hormone levels, and the Kupperman Index, a scale of self-reported menopause symptoms.
During the 24-week study, the women received placebo or a dried dong quai extract (standardized to 0.5 mg/kg of ferulic acid) at a dose of three 500 mg capsules three times daily (total daily dose of 4.5 grams of root).[58] The study demonstrated that dong quai did not promote endometrial proliferation or increased maturation of vaginal epithelial cells, both common estrogenic effects. Compared with baseline, the level of symptoms decreased in the placebo and dong quai-treated groups, but no statistically significant difference was demonstrated between the two groups. Dong quai was well tolerated and no serious side effects were noted.
As this well-designed study demonstrated, dong quai is not an effective herbal medicine for menopause, despite its popularity for this use in the West.[58] In fact, a standard text of the Chinese materia medica considers dong quai to be contraindicated for yin deficiency with heat signs, a classical Chinese description of menopause.[51]
When used in a more traditional manner, in a formula and for dysmenorrhea, dong quai has been shown to be a useful remedy. Kotani et al.[59] evaluated dong quai in a kampo (a traditional Japanese herbal medicine) for relief of dysmenorrhea in 41 women. This preparation was based on a traditional Chinese formula and contained six additional herbs: Angelica root, Paeoniae root, Hoelen, Astractylodes lanceae rhizome, Alismatis rhizome, and Cnidii rhizome. Patients were observed for two cycles to determine a baseline; they then received either the kampo formula or placebo for two cycles and subsequently were observed for two additional cycles with no medication. All women were allowed to take a nonsteroidal anti-inflammatory drug (diclofenac sodium) as needed for pain management. At the sixth month, dysmenorrhea was significantly less in the treatment group (P = .05 versus placebo), as measured by self-questionnaire and a visual analogue scale for pain.

Figure 3. Dong quai. Reprinted from: Chinese Herbal Medicine: Materia Medica, with permission from Eastland Press, P.O. Box 99749, Seattle, Wash. 98199. Copyright 1993. All rights reserved
Considerations for Patient Use
Despite its reputation, dong quai is not an effective treatment for menopausal symptoms when used as a single agent. When used in a traditional multiple-herb formulation, dong quai may have some efficacy for treatment of PMS. Caution should be exercised when using dong quai with anticoagulants and, possibly, antiplatelet aggregating agents, because of the potential for interaction and increased risk of bleeding.[53,60] Traditional Chinese texts also advise against using this herb in the presence of an acute infection, such as a cold or flu.[51]
Black Cohosh
Black cohosh, Cimicifuga racemosa (L.) Nutt., is an indigenous North American plant with a long history of traditional use among Native American peoples. This phytomedicine has been used for a wide variety of indications, such as snakebite, malaria, and relief of many types of pain, including dysmenorrhea, childbirth, and rheumatism. During the 19th century, an alcohol extract of the root was adopted as a popular remedy by the leading herbal physicians of the day, known as the Eclectics, and it became a primary remedy for menstrual and climacteric complaints. In fact, black cohosh was one of the major ingredients in Lydia Pinkham's famous women's tonic, which sold widely for more than 50 years. Knowledge of this useful herb was passed through the Eclectics to Europe, where black cohosh remains in favor to this day, especially as a treatment for menopausal symptoms.[61,62] This herb should not be confused with blue cohosh; despite their similar common names, they are completely different plant species.
Although the mechanism of action of black cohosh has not been fully elucidated, triterpene glycosides or saponins are thought to be the main active constituents. These include the xylosides actein, 27-deoxyactein, and cimicifugoside.[63] Other potentially important constituents include formononetin, a weak isoflavone, and organic acids, including isoferulic and salicylic acids.[64] The effects of black cohosh extract on hormone levels in animal models and humans are complex and not always consistent.[65-67] Binding to estrogen receptors can be demonstrated in animal models, but in humans the lipophilic fractions (which do not contain the cytosolic estrogen receptors) are thought to contain the active constituents. These constituents appear to reduce levels of luteinizing hormone, but not follicle-stimulating hormone, prolactin, or estrogen.[5,68] The strongest evidence for the use of black cohosh root comes from the results of clinical trials.
Clinical studies
Most of the clinical trials have been conducted using a proprietary formula, Remifemin (Shaper & Brummer), standardized to contain 1 mg triterpene glycosides, measured as 27-deoxyactein.[69] A series of open clinical studies, conducted with more than 800 subjects, demonstrated good efficacy of black cohosh root extract (BCRE) in relieving menopausal symptoms, such as vasomotor instability, with little or no toxicity.[70-73] Stoll[74] conducted a double-blind, placebo-controlled trial with 80 postmenopausal women that compared BCRE 8 mg/day with conjugated estrogen 0.625 mg/day or placebo. At the end of the trial, the BCRE-treated group showed significant improvement relative to placebo in the Kupperman Index, the Hamilton Anxiety Scale, and the maturation index of vaginal epithelium. It is important to note that, contrary to expectations, the estrogen-treated group showed no difference from placebo. Therefore, the findings of this study should be viewed with caution.
A recent double-blind, multicenter, controlled trial, sponsored by the manufacturer of a BCRE, followed 152 women with menopausal symptoms who received low-dose BCRE (Remifemin 2 mg/day) or high-dose BCRE (Remifemin 4 mg/day) for 12 weeks.[75] No placebo group was included, but comparison was made to a historical placebo control, a less-than-optimal study design. At 2, 4, 8, and 12 weeks, patients were assessed with the Kupperman Index, the Self-Rating Depression Scale, the Clinical Global Impression Scale, vaginal maturation index, and measurement of luteinizing hormone, follicle-stimulating hormone, estradiol, and sex hormone binding globulin.[75]At the end of therapy, approximately 80% of the patients and physicians rated BCRE as good to very good in reducing menopausal symptoms. No significant differences in the efficacy of the two dosage regimens were found, and no adverse effects were reported in either group. No significant differences in the hormonal parameters or vaginal epithelial maturation were reported, contrary to some other studies cited.[69]
Because of its affinity for estrogen receptors in some animal models, the safety of BCRE with respect to breast cancer risk has been questioned. This issue was addressed in two studies in human breast cell lines, neither of which has been published in the peer-reviewed literature.[69,76] The first, performed by the manufacturer of Remifemin, showed that BCRE in concentrations from 10-4 to 10-10 M did not stimulate growth proliferation in an estrogen-sensitive breast cancer cell line (MDA MB 435S).[69] Furthermore, when tamoxifen was added to the BCRE-treated cell line, greater inhibition in cell growth was seen than with tamoxifen alone, suggesting that BCRE had an anti-estrogenic effect in this model. Similarly, Freudenstein and Bodinet[76] reported a lack of stimulation by BCRE on the proliferation of MCF-7 cells, another estrogen-sensitive breast cancer cell line.

Figure 4. Black cohosh. Courtesy of New York State Museum
Considerations for Patient Use
Based on the clinical experience in Germany and the few controlled studies available, BCRE is an effective treatment for menopausal complaints, especially when estrogen replacement therapy is refused by the patient or not recommended on clinical grounds.[3,77-79] The German Commission E, an expert panel commissioned by the German government to assess herbal products, does not recommend that BCRE be continued for longer than 6 months, because of the lack of long-term safety data.[77] In popular use, however, this precaution is not always observed.
The recommended dose of Remifemin is 1 tablet, standardized to contain 20 mg of herbal drug, twice daily.[69] Other dosage forms are available and dosage depends on the method of preparation.[63]
Herbal Medications and pregnancy
Many patients and their providers are concerned about the safety of using herbal products during pregnancy and lactation. The pertinent safety data are limited and based on traditional use, animal studies, and knowledge of the pharmacologic activities of product constituents. Herbs that are generally considered toxic are absolute contraindications in pregnancy. (Many of these herbs are no longer in common use and therefore are not included in Table 1.) During pregnancy, herbs or herbal constituents that affect uterine contraction are not recommended. This warning is straightforward for herbs such as black cohosh, which are known to cause uterine contractions, and for herbs such as rue, tansy, or pennyroyal, which have been used traditionally as abortifacients. It may be surprising that the cathartic laxative herbs are contraindicated, but in therapeutic doses they can cause contraction of the smooth muscle of the uterus, just as they can cause cramping in the smooth muscle of the gut.[49,77,80-82]
It should also be noted that some herbs on the pregnancy warning list are commonly found in food: sage, tumeric, and garlic. At normal culinary doses, these herbs should not cause concern; they pose a potential risk only if taken in large doses or in a concentrated form. Ginger, used as an antiemetic in early pregnancy, is a good example. Ginger is a common food in Asian countries, where women regularly consume large amounts without apparent adverse effects. However, it has been noted that ginger is a potential emmenagogue, a traditional remedy that "brings on a period."[82] Emmenagogues are generally not considered to be as strong stimulants of uterine smooth muscle as abortifacients, and therefore most herbalists recommend caution rather than outright stricture with regard to ginger use during pregnancy.
A placebo-controlled clinical trial was conducted in 27 pregnant women using a dose of 1 gram of dried ginger root daily.[83] One patient had a spontaneous miscarriage that was not attributed to the adverse effects of ginger; no other adverse effects were recorded. The study also demonstrated significant efficacy of ginger as an antiemetic (P = .003 versus placebo). Despite the apparent safety demonstrated in this study and by food use in Asian countries, some experts recommend caution in the use of ginger during pregnancy, because of its effects on testosterone binding and thromboxane synthetase activity.[49,84] To balance efficacy with safety, it seems reasonable to permit use of ginger during pregnancy at low doses (not to exceed 1 gram daily), although not all experts agree.[3,49,77,78,80-82,85]
Herbal Medications and Lactation
Many herbal constituents are excreted in breast milk and thus are transmitted from the mother to the baby during breastfeeding. Herbalists recommend that mothers limit ingestion of those constituents that might have an adverse effect on the child (see Table 2). Herbs that are central nervous system stimulants, cathartic laxatives, highly toxic in general, or that contain potentially toxic essential oils are not recommended during lactation.[3,49,77,78,80-82,85]
Numerous herbs have been used in folk and traditional systems of healing to affect the flow of milk, but few, if any, scientific studies have looked at these agents for this indication[3,77,78,80,85,86] (see Table 3). A comprehensive review of these herbs has been published and combines pharmacologic data with ethnobotanical sources.[87]
Traditional and Folk Uses
Many other herbs have been cited in traditional sources for a wide variety of health complaints in women. Although the scientific literature on these herbs is generally scanty, they are routinely used in herbal practice, and at least a few deserve mention.
Dandelion leaf (Taraxacum officinale GH Weber ex Wigg.) is often cited as an effective diuretic, especially for PMS-related water weight gain.[80] A wide variety of herbs have been used for dysmenorrhea, including one better known as a sedative, valerian (Valeriana officinalis L). An antispasmodic effect on uterine smooth muscle has been described for valerian.[81] Other uterine relaxants and/or tonics in common use include wild yam (Dioscorea villosa L.), motherwort (Leonurus cardiaca L.), and two closely related species, black haw and crampbark (Viburnum prunifolium L. and Viburnum opulus L.).[77,80,81]
In common herbal practice, each of these herbs would be shaded for its own particularities. For example, wild yam is often used when inflammation is also present and motherwort when cardiac symptoms accompany menstrual complaints. The Viburnum species are especially useful as pain relievers, because they contain salicylic acid in potentially therapeutic doses and muscle relaxants.[81] Given the complexity of the traditional and ethnobotanical data available, much interesting work remains to be done to evaluate these herbs scientifically.
Conclusion
A rich pharmacopoeia of herbal remedies exists for many conditions of special interest to women. Many of these herbs have traditional uses dating back thousands of years and spanning cultures as diverse as ancient Egypt, China, India, and Native American tribes. For a select number of herbs, investigators are elucidating the constituents, mechanism of action, and therapeutic activities through studies using tissue culture, animal models, and human trials. Safety data, especially during pregnancy and lactation, are still largely lacking, and recommendations are not uniform among experts. However, with reasonable caution, a great deal of good can be done for women with phytomedicines, and with continued research in this area, much more good will follow.
Acknowledgements
The author wishes to thank Jean Wallace, PhD, for her editorial assistance in developing this article.
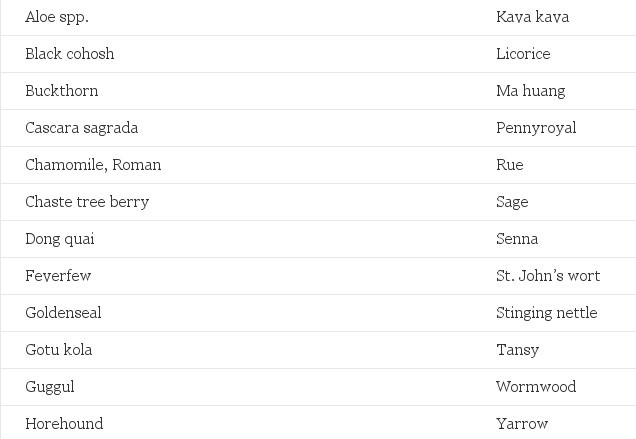

Table 1. Common Herbs to Avoid in Pregnancy
- Aloe spp.
- Black cohosh
- Buckthorn
- Cascara sagrada
- Cocoa
- Coffee
- Kava kava
- Ma huang
- Sage
- Senna
- Tea
- Wintergreen
Use with caution
- Cocoa
- Coffee
- Tea
Avoid excessive consumption relative to usual and customary food use.
Source: References 49,77,80-82.
Table 2. Common Herbs to Avoid While Breast-Feeding
Herbs that promote milk flow -
- Caraway
- Celery root and seed
- Chaste tree berry
- Fennel
- Fenugreek
- Goat's rue
- Raspberry
- Rauwolfia
- Verbena
Herbs that reduce milk flow -
- Castor bean
- Jasmine flower
- Sage
Source: References 3,77,78,80,85,86.
Table 3. Herbs Traditionally Used to Affect Milk Production
© Mary L. Hardy. J Am Pharm Assoc 40(2):234-242, 2000.
© 2000 American Pharmaceutical Association, Inc.
References
- Brooke E. Women Healers: Portraits of Herbalists, Physicians, and Midwives. Rochester, Vt: Healing Arts Press; 1995.
- Grieve M. A Modern Herbal. London: Cresset Press Book; 1973.
- Weiss RF. Herbal Medicine. Gothenburg, Sweden: AB Arcanum; 1988.
- Oil of Evening Primrose. Monograph. Lawrence Review of Natural Products. Levittown, Pa: Pharmaceutical Information Associates; 1993.
- Schulz V, Hänsel R, Tyler VE. Rational Phytotherapy: A Physician's Guide to Herbal Medicine, 3rd ed. Berlin: Springer-Verlag; 1998.
- Horrobin DF, Manku MS, Brush M, et al. Abnormalities in plasma essential fatty acid levels in women with premenstrual syndrome and with non-malignant breast disease. J Nutr Med. 1991;2:259-64.
- Brush M, Watson J, Horrobin D, et al. Abnormal essential fatty acid levels in plasma of women with premenstrual syndrome. Am J Obstet Gynecol. 1984;150:363-6.
- Gateley CA, Maddox PR, Pritchard GA, et al. Plasma fatty acid profiles in benign breast disorders. Br J Surg. 1992;79:407-9.
- Cameron I, Fraser I, Smith S. Clinical Disorders of the Endometrium and Menstrual Cycle. Oxford: Oxford University Press; 1998.
- Brush MG. Evening primrose oil in the treatment of premenstrual syndrome. In: Horrobin DF, ed. Clinical Uses of Essential Fatty Acids. Montreal: Eden Press; 1982:155-62.
- Larson B, Jonasson A, Fianu S. Evening primrose oil in the treatment of premenstrual syndrome. Curr Ther Res. 1989;46:58-63.
- Ylikorkala O, Puolakka J, Makarainen L, et al. Prostaglandins and premenstrual syndrome. Prog Lipid Res. 1986;25:433-5.
- Khoo SK, Munro C, Battistutta D. Evening primrose oil and treatment of premenstrual syndrome. Med J Australia. 1990;153:189-92.
- Budeiri D, Li Wan Po A, Dornan JC. Is evening primrose oil of value in the treatment of premenstrual syndrome? Control Clin Trials. 1996;17:60-8.
- Brush MG. Efamol (evening primrose oil) in the treatment of the premenstrual syndrome. In: Horrobin DF, ed. Clinical Uses of Essential Fatty Acids. London: Eden Press, Inc; 1982.
- Ockerman PA, Glans SA, Rassner S. Evening primrose oil as a treatment of premenstrual syndrome. Curr Ther Res. 1986;46:58-63.
- Pye JK, Mansel RE, Hughes LE. Clinical experience of drug treatment for mastalgia. Lancet. 1985;ii:373-7.
- Wetzig NR. Mastalgia: A 3 year Australian study. Aust N Z J Surg. 1994;64:329-31.
- BeLieu RM. Mastodynia. Obstet Gynecol Clin North Am. 1994;21:461-77.
- Genolet PM, Delaloye JF, DeGrandi P. Diagnosis and treatment of mastodynia [French]. Revue Medicale de la Suisse Romande. 1995;115:385-90.
- Gateley CA, Maddox PR, Mansel RE, et al. Mastalgia refractory to drug treatment. Brit J Surg. 1990;77:1110-2.
- Gateley CA, Mansel RE. Management of the painful and nodular breast. Brit Med Bull. 1991;47:284-94.
- Gateley CA, Meirs M, Mansel RE, et al. Drug treatments for mastalgia: 17 years experience in the Cardiff Mastalgia Clinic. J Roy Soc Med. 1992;85:12-5.
- Pain JA, Cahill CJ. Management of cyclical mastalgia. Brit J Clin Pract. 1990;44:454-6.
- Pashby N, Mansel R, Hughes L, et al. A clinical trial of evening primrose oil in mastalgia. Brit J Surg. 1981;68:801-24.
- Cambell EM, Peterkin D, O'Grady K, et al. Premenstrual symptoms in general practice patients. Practice and treatment. J Reprod Med. 1997;42:637-46.
- Cotterell J, Lee A, Hunter J. Double-blind cross-over trial of evening primrose oil in women with menstrually-related irritable bowel syndrome. In: Horrobin D, ed. Omega-6 Essential Fatty Acids: Pathophysiology and Roles in Clinical Medicine. New York, NY: Wiley-Liss; 1990.
- Strid J, Jepson J, Moore V, et al. Evening primrose oil or other essential fatty acids for premenstrual syndrome. Cochrane Database of Systematic Reviews. 1999;2.
- Chenoy R, Hussain S, Tayob Y, et al. Effect of oral gamolenic acid from evening primrose oil on menopausal flushing. Br Med J. 1994;308:501-3.
- Brown DJ. Herbal research review: Vitex agnus castus clinical monograph. Q Rev Nat Med. Summer 1994;111-21.
- Snow JM. Vitex agnus-castus L. (Verbenaceae). Protocol J Botanical Med. 1996;Spr;1:20-3.
- Gomaa CS, El-Moghazy MA, Halim FA, et al. Flavonoids and iridoids from Vitex agnus-castus. Planta Medica. 1978;33:277.
- Chaste tree [monograph]. The Review of Natural Products. St. Louis, Mo: Facts & Comparisons; 1998.
- Du Mee C. Vitex agnus-castus. Aust J Med Herbalism. 1993;5:63-5.
- Jarry H, Leonhardt S, Wuttke W, et al. Agnus castus als dopaminerges wirkprinzip in mastodynon. Z Phytother. 1991;12:77-82.
- Jarry H, Leonhardt S, et al. In vitro prolactin but not LH and FSH release is inhibited by compounds in extracts of Agnus castus: direct evidence for a dopaminergic principle by the dopamine receptor assay. Exp Clin Endocrinol. 1994;102:448-54.
- Wuttke W, Gorkow C, Jarry J. Dopaminergic compounds in Vitex agnus castus. In: Loew D, Rietbrock N, eds. Phytopharmaka in Forschung und klinscher Anwendung. Darmstadt: Verlag; 1995.
- Sliutz G, Speiser P, Schultz AM, et al. Agnus castus extracts inhibit prolactin secretion of rat pituitary cells. Horm Metab Res. 1993;25:253-5.
- Mayo J. Premenstrual syndrome: a natural approach to management. Clin Nutr Insights. 1997;5:1-8.
- Halbreich U. Serum prolactin in women with pre-menstrual syndrome. Lancet. 1976:654-6.
- Böhnert KJ. The use of Vitex agnus castus for hyperprolactinemia. Quart Rev Nat Med. 1997:19-21.
- Loch E, Böhnert KJ, Peeters M, et al. The treatment of menstrual disorders with Vitex agnus-castus tincture. Der Frauenarzt. 1991;32:867-70.
- Dittmar F, Böhnert KJ, Peeters M, et al. Premenstrual syndrome: treatment with a phytopharmaceutical. Therapiewoche Gynäkol. 1992;5: 60-8.
- Peteres-Welter C, Albrecht M. Menstrual abnormalities and PMS. Vitex agnus-castus in a study of application. Therapiewoche Gynäkol. 1994;7:49-52.
- Milewicz A, Gejdel E, Sworen H, et al. Vitex agnus castus extract in the treatment of luteal phase defects due to latent hyperprolactinemia. Results of a randomized placebo-controlled double-blind study. Arzneimittel-Forschung. 1993;43:752-6.
- Propping D, Katzorke T, Belkien L. Diagnosis and therapy of corpus luteum deficiency in general practice. Therapiewoche. 1988;38:2992-3001.
- Laurtzen C, Reuter HD, Repges R, et al. Treatment of premenstrual tension syndrome with Vitex agnus castus. A controlled, double-blind study versus pyridoxine. Phytomedicine. 1997;4:183-9.
- Cahill DJ, Fox R, Wardle PG, et al. Multiple follicular development associated with herbal medicine. Hum Reprod. 1994;9:1469-70.
- McGuffin M, Hobbs C, Upton R, et al. American Herbal Products Association's Botanical Safety Handbook: Guidelines for the safe use and labeling for herbs of commerce. Boca Raton, Fla: CRC Press; 1997.
- Klepser T, Nisly N. Chaste tree berry for premenstrual symptoms. Altern Med Alert. 1999:64-7.
- Bensky D, Gamble A, Kaptchuk T. Chinese Herbal Medicine: Materia Medica. Seattle, Wash: Eastland Press; 1986:474-6.
- Bone K. Clinical Applications of Ayurvedic and Chinese Herbs. Warwick, Queensland, Australia: Phytotherapy Press; 1996.
- Huang KC. The Pharmacology of Chinese Herbs. Boca Raton, Fla: CRC Press; 1993.
- Zhu D. Dong quai. Am J Chin Med. 1987;15:117-25.
- Noé J. Angelica sinensis: A monograph. J Naturopathic Med. 1997;winter 1997:66-72.
- Belford-Courtney R. Comparison of Chinese and Western uses of Angelica sinensis. Aust J Med Herbalism. 1993;5:87-91.
- Sheu SJ, Ho YS, Chen YP, et al. Analysis and processing of Chinese herbal drugs. IV. The study of Angelicae radix. Planta Medica. 1987;53:377-8.
- Hirata JD, Swiersz LM, Zell B, et al. Does dong quai have estrogenic effects in postmenopausal women? A double-blind, placebo-controlled trial. Fertil Steril. 1997;68:981-6.
- Kotani N, Oyama T, Hashimoto H, et al. Analgesic effect of a herbal medicine for treatment of primary menstrual dysmenorrhea: a double-blind study. Am J Chinese Med. 1997;25:205-12.
- Shimuzu M, Matsuzawa T, Suzuki S, et al. Evaluation of Angelica radix (Touki) by the inhibitory effect on platelet aggregation. Chem Pharm Bull. 1991;39:2046-8.
- Gruenwald J. Standardized Black Cohosh (Cimicifuga) Extract Clinical Monograph. Q Rev Nat Med. 1998;summer:117-25.
- Foster S. Black cohosh: A literature review. Herbalgram. 1999;Winter:35-49.
- Beuscher N, Clay A, Reichert R. Cimicifuga racemosa L.- Black Cohosh. Zeitschrift fur Phytotherapy. 1995;16:301-10.
- Black cohosh [monograph]. Lawrence Review of Natural Products. 1992;April.
- Einer-Jensen N, Zhao J, Andersen K, et al. Cimicifuga and Melbrosia lack oestrogenic effects in mice and rats. Maturitas. 1996;25:149-53.
- Düker E, Kopanski L, Jarry H, et al. Effects of extracts from Cimicifuga racemosa on gonadotropin release in menopausal women and oophorectomized rats. Planta Med. 1991;57:420-4.
- Jarry H, Harnischfeger G. Studies on the endocrine effects of the contents of Cimicifuga racemosa: 1. Influence on the serum concentration of pituitary hormones in ovariectomized rats. Planta Med. 1985 Feb;(1)1:46-9.
- Jarry H, Harnischfeger G, Düker E. Studies on the endocrine effects of the contents of Cimicifuga racemosa: 2. In vitro binding of compounds to estrogen receptors. Planta Medica. 1985 Aug;(4):316-9.
- Scientific Brochure of Remifemin [English translation]. Salzgitter, Germany: Schaper & Brummer; 1997.
- Petho A. Climacteric complaints are often helped with black cohosh. Arztliche Praxis. 1987;47:1551-3.
- Stolze H. Another way to treat climacteric complaints. Gynecologie. 1982;1:14-6.
- Vorberg G. Therapy of climacteric complaints. Zeitschrift für Allegemeinmedizin. 1984;60:626-9.
- Warnecke G. Influence of a phytopharmaceutical on climacteric complaints. Die Medizinische Welt. 1985;36:871-4.
- Stoll W. Phytopharmaceutical influences on atrophic vaginal epithelium. Double-blind study on Cimicifuga versus an estrogen preparation. Therapeutikon. 1987;1:23-32.
- Liske E, Wüstenberg P. Therapy of climacteric complaints with Cimicifuga racemosa: herbal medicine with clinically proven evidence. Menopause. 1998;5:250.
- Freudenstein J, Bodinet C. Influence of an isopropanolic aqueous extract of Cimicifuga racemosae rhizoma on the proliferation of MCF-7 cells. 23rd International LOF-Symposium on Phytoestrogens. University of Ghent, Belgium; 1999.
- Blumenthal M, Busse WR, Goldberg A, et al. The Complete German Commission E Monographs. Austin, Tex: American Botanical Council; 1998.
- Bisset NG, Wichtl M. Herbal Drugs and Phytopharmaceuticals. Boca Raton, Fla: CRC Press; 1994.
- Lieberman S. Black cohosh for menopausal symptoms. J Women's Health. 1998;7:525-9.
- Greunwald J, Brendler T, Jaenicke C. PDR for Herbal Medicines. Montvale, NJ: Medical Economics Company, Inc; 1998.
- Brinker F. A comparative review of eclectic female regulators. J Naturopathic Med. 1997;7:11-25.
- Brinker F. Herb Contraindications and Drug Interactions. Sandy, Oreg: Eclectic Institute, Inc; 1997.
- Fischer-Rasmussen W, Kjaer S, Dahl C, et al. Ginger treatment for hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol. 1990;38:19-24.
- Backon J. Ginger in preventing nausea and vomiting of pregnancy; a caveat due to its thromboxane synthetase activity and effect on testosterone binding. Eur J Obstet Gynecol Reprod Biol. 1991;42:163-4.
- Yarnell E. Botanical medicine in pregnancy and lactation. Altern & Compl Ther. 1997:93-100.
- Miller L, Murray W. Herbal Medicinals: A Clinicians Guide. Binghampton, NY: Haworth Press; 1998.
- Bingel A, Farnsworth N. Higher plants as potential sources of galactagogue. Econ Med Plant Res. 1991;6:1-54.
Comments
comments powered by Disqus