Authors - Henk Asscheman, MD and Louis J.G. Gooren, MD
Henk Asscheman and Louis J.G. Gooren are both affiliated with the Division of Andrology/Endocrinology, Free University Hospital, Amsterdam. Please send all correspondence to: Professor Louis J.G. Gooren, Free University Hospital, P0. Box 7057, 1007 MB Amsterdam, the Netherlands.
© 1992 by The Haworth Press, Inc. All rights reserved.
Introduction
Except for the sex chromosomes and gonads all bodily differences between men and women must be attributed to the actions of sex hormones. While the inherent tendency of the prenatal human organism is to develop along female lines, prenatal differentiation as a male depends on testicular hormones (Mullerian-inhibiting hormone and testosterone and its derivates). The wider bony pelvis in girls in comparison with boys, is probably dependent on local effects of prenatal ovarian estrogen production. There is no known fundamental difference in sensitivity to the biological action of sex steroids on the basis of the genetic patterns of 46,XY and 46,XX.
The prepubertal period is hormonally relatively quiescent (Conte, Grumbach, Kaplan & Reiter, 1980). The hormones of puberty accentuate sex differences. Testosterone and its potent derivate 5alpha-dihydrotestosterone (DHT) induce penile growth and secondary sex characteristics as sexual hair, deepening of the voice, a muscular build and the greater average height in males in comparison to the females. In girls, estrogens in conjunction with progestagens induce breast formation and a fat distribution predominantly around the hips; subcutaneous fat padding produces a softness of the body configuration and of the skin. The skin in women is further generally less oily than in men; the latter on the basis of activation of the sebaceous glands by androgens.
Fundamental to sex reassignment treatment is the acquisition to the fullest extent possible of the sex characteristics of the other sex. With the exception of the internal and external genitalia, these characteristics are contingent of the biological effects of the respective sex steroids. Therefore (semi)synthetic sex steroids are indispensable tools in sex reassignment treatment. The use of cross-gender hormone treatment is associated with a better outcome (Hamburger, 1969; Leavitt et al., 1980).
The "two year real-life test" (Money & Ambinder, 1978) is pivotal in diagnostic-therapeutic approach of gender dysphoria. It allows both the gender-dysphoric subject and the psychologist/physician to examine whether sex reassignment relieves the burden of gender dysphorla. The emerging physical changes associated with cross-gender sex hormone treatment will facilitate the assumption of the role as a member of the other sex both in private life and in society.
The attempt to induce cross-gender sex characteristics in transsexuals-generally biologically normally differentiated males and females in their adult years-can be subdivided into two aspects:
- Annihilation of sex characteristics of the original sex.
- Induction of sex characteristics of the sex one reckons oneself to belong to.
- Unfortunately, the annihilation of sex characteristics of the original sex is incomplete. In male-to-female transsexuals, there is no mode of treatment to revert earlier effects of androgens on the skeleton. The greater height, the shape of the jaws, the size and shape of the hands and feet, and the narrow width of the pelvis can not be redressed once they have reached their final size at the end of puberty. Conversely, the relative lower height in female-to-male transsexuals (in the Netherlands an average of 12 cms) and the broader hip configuration will not change under the influence of hormonal treatment.
- While in the majority of female-to-male transsexuals, a complete and inconspicuously masculine development can be induced with androgenic hormones, the effects of feminizing hormone treatment in male-to-female transsexuals can be objectively unsatisfactory with regard to reduction of male-type of facial/beard hair and induction of breast development.
Transsexuals often expect and sometimes demand rapid and complete changes immediately after the start of the hormonal therapy. The induced effects of cross-sex hormones are, however, limited and appear only gradually. Before starting hormone treatment a clear discussion of the possible changes and the limits in an individual patient, is indispensable in order to prevent unrealistic expectations. In the next and following sections we describe the effects of cross-gender hormones separately for male-to-female and female-to-male transsexual subjects.
Which Hormones and Which Dose?
For each of the above mentioned aspects of hormone treatment exists a large array of (semi) synthetic sex steroids. There are no solid literature data to prove certain hormonal drugs superior in efficacy to others. Only two published studies give an indication of the value of different hormone schedules in the treatment of transsexuals, but the results are far from conclusive (Meyer et al., 1981 & 1986). The choice of hormonal drugs in the treatment of transsexualism depends on availability (national regulations, pharmaceutical marketing), local traditions, side effects, route of administration, cost and folk belief (in particular from the side of the transsexual subject and his/her peer group, but also from the physician). Optimal dosages of these drugs have not yet been established.
The first effects of the cross-gender sex hormones appear already after 6 to 8 weeks (Futterweit, 1980). Voice changes in female-to-male transsexuals and the development of painful breast noduli in male-to-female transsexuals are the first manifestations. Thereafter the changes take over 6 to 24 months and even longer before they are complete (beard growth may take 4 to 5 years in androgen-treated female-to-male transsexuals).
Cross-Sex Hormone Treatment in Male-to-Female Transsexuals
Annihilation of Male Characteristics
In male-to-female transsexuals suppression of the original sex characteristics can be obtained by compounds that exert directly or indirectly an antiandrogenic effect. Androgens are for their production dependent on stimulation by the pituitary hormone luteinizing hormone (LH) which, in turn, is stimulated by the hypothalamic hormone luteinizing hormone-releasing hormone (LHRH). The biological action of androgens is contingent on their interaction with hormone receptors in the body's tissue cells. Interference with any of these mechanisms will lead to a decline of the biological action of androgenic hormone. Some of the drugs that will be listed have a dual action in this respect (Table 1).
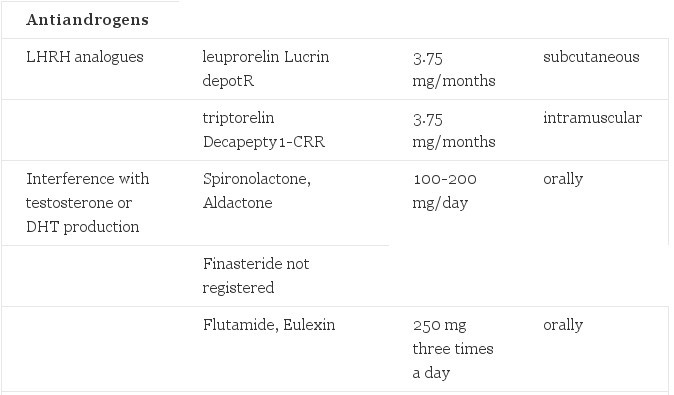
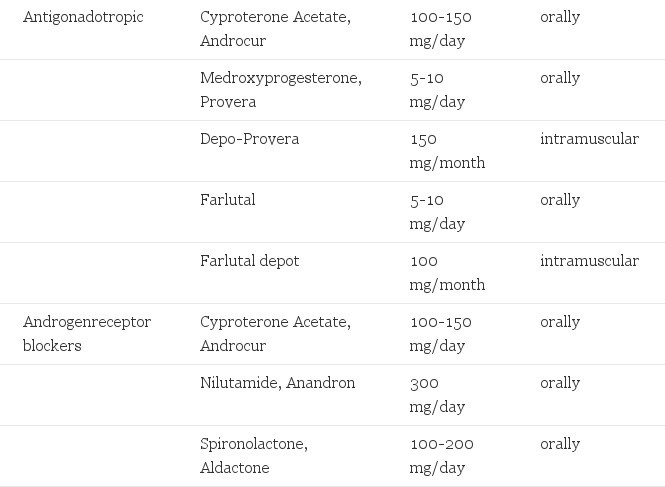
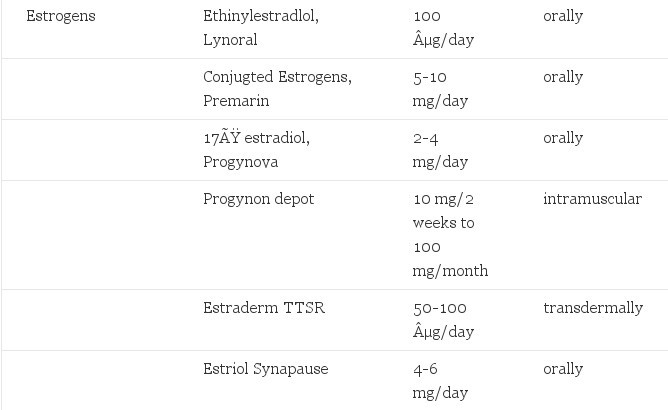
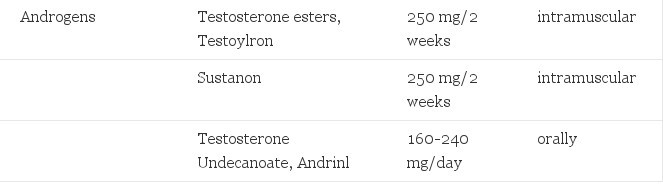
TABLE 1. Hormones Used in Cross-Gender Hormone Treatment of Transsexualism
- Suppression of gonadotropins (the pituitary hormones) that stimulate testicular and ovarian hormone production can be achieved by LHRH analogues: triptorelin and leuprorelin 3.75 mg/4 weeks are available as injectables. Their cost is prohibitive; a major side effect is hot flashes of the type that postmenopausal women experience. There is no reported experience with these drugs in transsexuals. Also cyproterone acetate, progestagens and high dose estrogens suppress gonadotropins by their negative feedback action. Progestagens are available in the form of medroxyprogesterone acetate (Provera) as a parenteral drug (150 mg/6 weeks) or oral drug (5-10 mg/day). They probably also interfere with the androgen receptor.
- Interference with the production of testosterone or its conversion to the potent metabolite 5alpha-dihydrotestosterone (DHT) can be exercised by drugs like spironolactone (Aldactone) 100-200 mg/day. Finasteride is a potent 5 alpha-reductase inhibitor preventing the conversion of testosterone to DHT and therewith reducing its biological effect.
- Drugs that interfere with receptor binding of androgens (or in the future with postreceptor mechanisms) have been used success-fully. Cyproterone acetate (Androcur) 100 mg/day orally or 300 mg/month intramuscularly and less effectively medroxyprogesterone acetate have also antigonadotropic action. The "pure" antiandrogens nilutamide (Anandron) 300 mg/day and flutamide (Eulexin 250 mg, three times a day) are potent drugs. They are less suited as monotherapy since by their interference with the negative feed-back action of androgens, they stimulate gonadotropin production and subsequently androgen production. Spironolactone has also receptor-blocking properties.
Of all the above drugs side-effects have been reported. Some are inherent in the interference with the biological action of androgens like a reduction of muscle mass and power and of the hemoglobin content. Some patients will complain of loss of energy and vitality to straightforward depression. The antiandrogen we have extensively used is cyproterone acetate (100mg/day). Side effects encountered are those mentioned above. Our alternative drug is spironolactone. Several studies have demonstrated its efficacy in transsexuals and in hirsute women alike. It has also an antihypertensive effect, since the drug was designed as a diuretic.
We have limited experience with nilutamide and LHRH antagonists. Medroxy-progesterone acetate has been widely used in the USA also in sex offenders. It is a satisfactory drug also in the view of the mild side effects and the costs. A randomized double-blind clinical trial to establish the best suited antiandrogen compound in transsexuals has not been performed so far.
Following orchiectomy we try to reduce or terminate antiandrogen therapy. Sexual hair growth is clearly dependent on androgens for its initiation and it would be logical to believe that antiandrogens are redundant following orchiectomy. Though not verified by research data, patients claim that also after orchiectomy their sexual hair growth is still reduced by antiandrogens. Due to the much shorter life cycle of sexual hair on the trunk, arms and legs as compared to the face and the greater density of hair follicles in the beard area, beard growth is not reduced to a cosmetically acceptable degree by antiandrogens. Other measures like depilation by electrolysis are needed. Those subjects who are young enough to have no significant beard development or whose racial background provides them with little or no beard development, are not in need of antiandrogens for this purpose.
Induction of Female Characteristics
The principal feminizing hormones are estrogens. Estrogens alone can induce most typical female characteristics as has been shown in cases of Turner syndrome in which the ovaries fall to produce hormones. A second sex steroid produced by the ovaries is progesterone. It prime function is to prepare the uterine mucosa for nidation. Its feminizing effect is probably limited, but effects of breast tissue have been described. Meyer et al. (1986) found no difference in breast hemicircumference between male-to-female transsexuals who had used estrogens only and those who had used both estrogens and progestagens, but this study was not a randomized double-blind clinical investigation.
It has been suggested that "unopposed action of estrogens" (by progestagens) would constitute a risk factor for carcinomas of the breast and there are epidemiological data in support of this. On the other hand studies in users of oral contraceptives have suggested that progestagens play a role in breast cancer development, though oral contraceptives overall are associated with a reduced risk of breast cancer. Three cases of breast carcinomas in male-to-female transsexuals have been published but it is difficult to arrive at statistically reliable conclusions on risks since the total number of users is unknown and no data are available on what estrogens and how long these three subjects have been taking this medication. Of note is the fact that breast carcinomas have not been observed in men with prostatic carcinoma taking high doses of estrogens but the follow-up may have been too short in view of their lethal disease. Male-to-female transsexuals should be informed about this risk factor. As with the general female population, they should receive information on self-examination of their breasts. At medical checkups their breasts should be physically examined and if palpation is suspect, mammography and eventually biopsy should follow. The fact that transsexuals have often breast implants may impede physical (self)examination of the breast. In our population of more than 500 hormonally treated male-to-female transsexuals (follow-up 0-20 years with an estimated median of 6.5 years) we have not come across a case of breast carcinoma.
In terms of estrogenic effects there is no superior estrogen. The choice depends mainly on availability, costs and the preference of the subject. Careful clinical studies on side effects in the form of randomized double-blind placebo-controlled studies with different estrogens are non-existent. The chemical formula and the route of administration lead to substantial differences in characteristics of estrogenic drugs. All oral estrogens first pass the liver after intestinal absorptidn and exert an effect on liver metabolism, evidenced by their effects on lipids, clotting factors and renin The liver metabolism of estrogen is also related to the chemical formula of the estrogen. Ethinyl estradiol is slowly metabolized whereas l7beta-estradiol is broken down rather rapidly, explaining the 10-20 fold difference in daily dosage. Concomitant drug use (anti-epileptics) may induce a more rapid metabolism of estrogens.
- Ethinyl estradiol (Lynoral), 50 µg orally twice (or more) daily, is the most potent estrogenic drug. It is a chemical modification of 17ß-estradiol, the main estrogen of the body, and it is slowly metabolized by the liver, but has a large effect on other metabolic pathways in the liver. It is very cheap, easily available worldwide and often used by male-to-female transsexuals because it can be obtained from women friends or without prescription in many countries as the oral contraceptive pill (always combined with progestagens). In the current estradiol assays its presence is not measured but the resulting suppression of gonadotropins suggests its use. A specific assay for ethinyl estradiol is commercially available.
- "Natural" estrogens, which are not natural but metabolized estrogens from other species (pregnant mare urine) are more appropriately called conjugated estrogens (Premarin and other brands). Active dose in postmenopausal women is 0.625-1.25 mg, but for cross-gender hormone therapy the active dose is 5-10 mg (Meyer et al., 1986). They are largely metabolized at first liver passage. It is said that they have less side effects than other estrogens. However, the supporting scientific evidence is very weak and in trials of secondary prevention of myocardial infarction in men, conjugated estrogens in a dose of 2.5 and 5 mg orally per day are clearly associated with an increased risk of thrombosis. Paradoxically, low doses appear to reduce the risk of cardiovascular disease in postmenopausal women.
- 17ß-Estradiol (or in short estradiol) is the most potent of the three forms of native estrogens in the human body. It is produced synthetically and can be administered orally (progynova, Estrofem, Zumenon 2-4 mg per day) metabolized in great part at first liver passage, intramuscularly (progynon-Depot 20-200 mg per month) or transdermally (Estraderm TISR 100 µg, patches are replaced twice weekly). In particular, this latter form is very promising because of its low number of estrogen-induced side effects.However, its efficacy in cross-gender hormone treatment has not been fully determined (a study is in progress at our clinic). A considerable number of patients (±10%) have skin problems at the application site and its sticking qualities can be a problem in patients that perspire easily or in a hot climate. Another obstacle is the price (US$ 1.00 per day), it is the most expensive estrogen therapy.
- Estriol (Synapause E3, Ovestin 2-6 mg orally per day) is a less potent native estrogen that is used in postmenopausal women for atrophic vaginitis and for urinary problems. In cross-gender hormone treatment high doses are necessary and estriol has no advantages over estradiol for this indication.
In our clinic ethinyl estradiol 100 µg orally per day has been the standard treatment for all male-to-female transsexuals until recently. With the introduction of transdermal estrogen we have changed our policy because of the frequent occurrence of thromboembolism in patients over 40 years of age (12%, Asscheman, Gooren & Eklund, 1989). All new male-to-female transsexuals older than 40 years are treated with Estraderm TTSR 100 µg two patches per week from the start. Younger patients are offered the same possibility but they are informed that their risk of thromboembolism is much lower (2.1%) and ethinyl estradiol 100 µg is proposed as an alternative. Intramuscular estrogen depots are not routinely given for two reasons. First, in case of side effects, not infrequent with estrogen therapy, it can take weeks before the serum levels of estradiol have normalized and second, male4o-female transsexuals tend to abuse estrogens under the wrong assumption "the more the better." We have seen subjects who used 800 mg progynon Depot, intramuscularly, per week with sometimes serious side effects (Gooren, Assies, Asscheman, de Slegte & van Kessel, 1988). With oral administration abuse is also not uncommon, but the doses are not so extreme.
After sex reassignment surgery we try to reduce the dose to a minimum that produces no clinical symptoms of sex hormone deficiency, but no lower than the minimum dose that protects against osteoporosis. In postoperative patients all kinds of estrogen substitution therapy are used depending on the personal preference of the transsexual patient and the lack of clinical symptoms of estrogen deficiency or side effects. Practically, this is similar to the estrogen treatment of postmenopausal women with some advantages: there is no need for progestagens and no risk of endometrial carcinoma.
Cross-Sex Hormone Treatment in Female-to-Male Transsexuals
Annihilation of Female Characteristics
The effects of estrogens on physical characteristics cannot be annihilated by antihormones. Antiestrogens administered to eugonadal women stimulate gonadotropin and subsequently ovarian hormone secretion. Theoretically, LHRH antagonists could be used. The objections have been mentioned earlier.
Transsexuals very much appreciate that their menstrual periods are terminated. This can be accomplished by progestagens with their antigonadotropic properties: medroxyprogesterone acetate (Provera, Farlutal) 5 mg 1 - 2 tablets/day or 150 mg intramuscularly/3 months, lynesterol (Orgametrji) 5 mg or norethisterone (Primolut N) 5 mg both 1 or 2 tablets/day. Androgens to be discussed in the next section have in high dosages also antigonadotropic action. There is no clear advantage in the combination of the two hormones unless androgens alone suppress menstrual bleeding insufficiently.
Induction of Male Characteristics
Androgens exert a powerful effect on the virilization process but completion may take as long as 24 years and sometimes even longer. The individual outcome depends on genetic factors both familial and racial. The degree of hairiness of siblings is a fair predictor of the virilization process.
To be used are testosterone esters 200-250 mg/2 weeks intramuscularly. Their brand names vary from place to place (Sustanon, Testoviron). As oral androgens testosterone undecanoate can be mentioned (Andriol) 160-240 mg/day, not available in the USA. With the latter preparation, menstrual bleeding is insufficiently suppressed in 50% of the patients and addition of a progestagen is required. The use of oral androgens with an alkyl group in the 17a position of the molecule is obsolete due to its hepatotoxicity. Oral androgens as mesterolone and fluoxymesterone are too weak for the induction of virilization.
In approximately 50-60% of the female-to-male transsexuals acne will occur. In 10-15% it is rather serious requiring dermatological treatment. It is now certain that androgen treatment has an unfavorable effect on the lipid profile. It places female-to-male transsexuals in the risk category of men. Therefore they must be advised not to smoke, to exercise moderately and to prevent over-weight and high blood pressure.
Effects of Cross-Gender Hormones in Male to-Female Transsexuals
Annihilation of the male pattern is possible for a number of secondary sex characteristics but only to a limited extent. Reduction of androgen-dependent hair growth with cyproterone acetate and ethinyl estradiol is fairly effective on the trunk and the limbs, but has a very limited success m the face. The Body hair does not disappear but following suppression of androgen-dependent growth, the hair becomes less coarse and less visible, resembling the vellus hair on the female Body in certain Body regions. If hairlessness of the Body is desired, only electrolysis is effective. Waxing and shaving can result in temporary hairlessness, which can be prolonged by the decrease in hair growth associated with estrogen and antiandrogen treatment. The beard hairs also become thinner and softer after several years of hormone use. Unfortunately, once the beard growth has fully developed and regular shaving is necessary, the result of antiandrogens alone is cosmetically unacceptable. Only electrolysis is effective in eliminating beard growth. In a few patients who had started treatment before developing visible hair growth, electrolysis could be avoided. After starting hormone treatment, male type scalp hair loss (masculine alopecia) ceases. Re-growth of scalp hair on bald areas is incomplete and of the vellus type. Hairstyle, hair implants or artificial hair techniques ("weaving," partial wigs) can successfully mask the masculine alopecia while hormones can at best make a minor contribution.
Penis length is not reduced by hormones, but due to its almost continuous flaccid state and an increase in lower abdominal fat, may appear reduced. Spontaneous erections are suppressed within 3 months but during erotic arousal erections still occur in the majority of our patients, evidencing the relative androgen-independence of this type of erection. Testicular volume is reduced by 25% within the first year of hormone use. This reduction is appreciated as a sign of progress and also makes hiding of the male genitals easier.
Induction of female characteristics is quite variable. In the initial phase of estrogen therapy, subareolar nodules which can be painful (Futterweit, 1980), are common. The breast size can be quantified by measuring the maximum hemicircumference over the nipple with a flexible ruler (either in the supine position or sitting which is our method). The increase in breast size evolves gradually with often periods of growth and periods of apparent standstill. The mean hemicircumference after 1 year is 10 cm in the supine position and 14 cm in the sitting position (the latter varies from 4 to 22 cm in our patients) and reaches its maximum after 18 to 24 months. In our patients the mean value is 18 cm, but it can vary from 4 to 28 cm. For comparison: in biological females it varies from 12 to 36 cm with a mean of 22 cm (own unpublished observations in a small number of these women). The values in male-to-female transsexuals are several centimeters less than in biological women. Moreover, the width of the male thorax is in general larger than that of the female thorax. Consequently, the proportional effect is judged as unsatisfactory by almost 50% of the male-to-female transsexual subjects. The majority of those unsatisfied request surgical breast implants. In more than 50% of the male-to-female transsexuals, the estrogen-induced breast size is judged as satisfactory by the transsexual subject herself, obviating breast surgery. In a small number of subjects unilateral or bilateral subcutaneous mastectomy has been performed because of pubertal gynecomastia. The hormonal effect on operated breasts is nil. In the latter cases early breast implants are indicated, but we prefer to wait at least one year before recommending any surgery including breast surgery.
In male-to-female transsexuals, estrogens do not affect the pitch of the voice, and a low voice can be a great handicap. Speech therapy is necessary to achieve a more feminine vocal range. Vocalcord surgery does not obviate the need for speech therapy in almost all cases, but the resulting higher pitched voice facilitates a female public presentation.
The subcutaneous and intra-abdominal fat distribution is sex steroid-dependent. Males preferentially accumulate fat in the upper abdomen ("apples") and females around the hips ("pears"). Estrogen treatment results generally in more fat around the hips but this is not the rule and can vary largely. Skeletal structures like jaws, size of hands and form of the pelvis do not change with the estrogen and/or antiandrogen treatment.
Not infrequently male-to-female transsexuals complain of a dry skin and fragile nails. This is a consequence of the reduction in sebaceous gland activity following antiandrogen treatment. Avoidance of detergents and application of ointment is mostly helpful.
Effects of antiandrogens alone or in combination with estrogens on the mood and the emotional functioning are often reported by our patients and their partners. Defmitive scientific proof in transsexuals that they are hormone-related is not available, but it is likely. In view of the consistency of these subjective reports and some studies in hypogonadal patients after substitution with appropriate hormones, an effect of hormones on the brain and consequently on brain functions like mood, is highly plausible.
Effects of Cross-Gender Hormones in Female-to-Male Transsexuals
Generally the virilization process proceeds subjectively and objectively in a satisfactory way and female-to-male transsexuals are pleased with it. It has no effect on their breast size. An oily skin and acne may become a problem. In comparison to other men they are rather short, but a short man is less conspicuous than a tall woman. The clitoris enlarges in all subjects though in a different degree. It sometimes suffices to have vaginal intercourse with a female partner, but that is not the rule. Most subjects indicate an increase of their libido following androgen treatment. Like male-to-female transsexuals female-to-male transsexuals must continue androgen treatment after ovariectomy to prevent hot flashes, loss of male characteristics and above all osteoporosis.
Side-effects of cross-gender hormones
Few systematic studies on side effects of cross-gender hormone treatment in transsexuals have been published. Meyer et al. (1986) found in 90 transsexuals only liver enzyme abnormalities and mild elevations of serum cholesterol and triglycerides. Case reports have described pulmonary embolism, cerebral thrombosis, myocardial infarction, prostatic metaplasia, and breast cancer in estrogen-treated male-to-female transsexuals and recurrent myocardial infarction in a female-to-male transsexual treated with androgens.
In 1989 we published a retrospective study on mortality and morbidity in 303 male-to-female and 122 female-to-male transsexuals who have been treated and followed at our clinic for 6 months to more than 13 years (Asscheman, Gooren & Eklund, 1989). Mortality in male-to-female transsexuals was 6-fold in-creased compared with the general population. This was in particular due to suicide and death by unknown cause. No deaths occurred in the female-to-male group but the median age was much lower. Side effects were common m the male-to-female transsexual patients. Significant increases were observed for venous thrombosis, pulmonary embolism, depressive mood changes, hyperprolactinemia and elevated liver enzymes in the male-to-female transsexual patients. In the female-to-male group acne (12.3%) and weight increases > 10% (17.2%) were the main side effects. Many side effects were reversible with appropriate therapy or temporary discontinuation of hormones.
The occurrence of serious side effects (e.g., the prevalence of thromboembolic disease of 2.1% in patients below 40 years of age and 12% in patients above 40 years) was, however, not rare. In view of the needs of transsexuals these side effects present a difficult dilemma in hormonal gender reassignment. At present no firm guidelines can be given. The cornerstone of the decision to prescribe cross-gender hormones remains with the explanation of the possible side effects to the patients and careful clinical judgment.
Efforts to reduce the risk of thromboembolic events by transdermal administration of estrogen are very promising but not conclusive at this moment. Further follow-up of this relatively young population to disclose long-term side effects is required.
Conclusion
Hormones are indispensable tools for the induction and maintenance of the characteristics of the sex the transsexual reckons him/herself to belong to. Following sex reassignment surgery they are hypogonadal and they must receive in principle lifelong hormone replacement in the same fashion as other hypogonadal patients. The main goal is to prevent future osteoporosis manifesting itself in the fifth and higher decades of their lives.
References
- Asscheman, H., Gooren, L.J.G. & Ekiund, P.L.E. (1989). Mortality and morbidity in transsexual patients with cross-gender hormone treatment. Metabolism, 38, 869-873.
- Conte, FA., Grumbach, M.M., Kaplan, S.L. & Reiter, E.O. (1980). Correlation of LRH-induced LH and FSH release from infancy to 19 years with the changing pattern of gonadotropin secretion in agonadal patients: Relation to the restraint of puberty. Journal of Clinical Endocrinology and Metabolism, 50, 163-168.
- Futterweit, W. (1980). Endocrine management of transsexuals. Hormonal profiles of serum prolactin, testosterone and estradiol. New York State Journal of Medicine, 80, 1260-1264.
- Gooren, L.J.G., Assies, J., Asscheman, H., de Slegte, R. & van Kessel, H. (1988). Estrogen-induced prolactinoma in a man. Journal of Clinical Endocrinology and Metabolism, 66, 444-6.
- Hamburger, C. (1969). Endocrine treatment of male and female transsexualism. In: Transsexualism and sex reassignment. Green, R. & Money, J. (eds), Baltimore, Johns Hopkins University Press, 291-307.
- Leavitt, F., Berger, J.C., Hoepnerr, J.A. & Northop, G. 1980). Presurgical adjustment m male transsexuals with and without hormonal treatment. Journal of Nervous and Mental Diseases, 168, 693-697.
- Meyer, W.J., Fmkelstein, J.W., Stuart, C.A., Webb, A., Smith, E.R., Payer, A.F. & Walker, PA. (1981). Physical and hormonal evaluation of transsexual patients during hormonal therapy. Archives of Sexual Behavior, 10, 347-356.
- Meyer, W.J., Webb, A., Stuart, C.A., Finkeistein, J.W., Lawrence, B., Walker, PA. (1986). Physical and hormonal evaluation of transsexual patients. A longitudinal study. Archives of Sexual Behavior, 15, 121-138.
- Money, J. & Ambinder, R. (1978). Two year real life diagnostic test: Rehabilitation versus cure. In: Controversy in psychiatry. Brady, J.P. & Brody, H.K.M. (eds). Philadelphia, W.B. Saunders, 833-845.
Comments
comments powered by Disqus