Cross-sex hormone treatment of transgender adults leads to very few long-term side effects, according to the authors of the largest study to date to examine this issue.
More than 2000 patients from 15 US and European centers participated in the retrospective study, called "Comorbidity and Side Effects of Cross-Sex Hormone Treatment in Transsexual Subjects", and nearly 1600 received at least 1 year of follow-up, the authors reported.
"Our results are very reassuring," principal investigator Henk Asscheman, MD, PhD, who heads HAJAP, his clinical research company in Amsterdam, the Netherlands, told Medscape Medical News. "There are mostly minor side effects and no new [adverse events] observed in this large population."
Speaking at ICE/ENDO 2014 last week, where he presented the initial results of the research, Dr. Asscheman said the data confirm findings from smaller studies published in the past decade.
"The take-home message," he said, "is that when using the guidelines from the Endocrine Society ’Endocrine Treatment of Transsexual Persons’ http://www.tgmeds.org.uk/doh-transgender-experiences.pdf, you are not going to see a lot of comorbidities with cross-sex hormone treatment."
Venous Thromboembolism Lower Than Prior Reports
The primary serious side effect, venous thromboembolism, occurred in 1% of persons undergoing male-to-female (MTF) transgender transition and was due to estrogen treatment.
Dr. Asscheman said although this incidence is still high, it is lower than reported in the past.
Among the 1596 adults who completed follow-up, 1073 were MTF and 523 were female to male (FTM). The MTF group had a mean follow-up of 5.6 years and a mean age of 35.0 years, and on average, the FTM group had a follow-up of 4.5 years and age of 27.5 years.
More than 70% of the MTF group received cyproterone acetate (in Europe) or spironolactone, as an antiandrogen, in addition to estrogen treatment, he noted.
Among FTM subjects, more than 90% received intramuscular or topical (gel) testosterone administration.
The diagnosis of "comorbidity," Dr Asscheman said, was made either by another medical professional or by a prescription for a relevant medication.
At entry into the study (baseline), the most common comorbidity in both groups was depression, with a 24.9% incidence in MTF subjects and 13.6% in FTM, according to Dr. Asscheman. He noted, however, that the frequency of depression varied greatly among the study centers.
Even after treatment, 26 (2.4%) of the MTF subjects and 7 (1.4%) of the FTM subjects still reported depression, leading Dr. Asscheman to tell the large audience, "Sex-reassignment treatment does not cure depression."
Other reported pretreatment comorbidities are shown in the table.
Comorbidities Before Transgender Hormonal Therapy
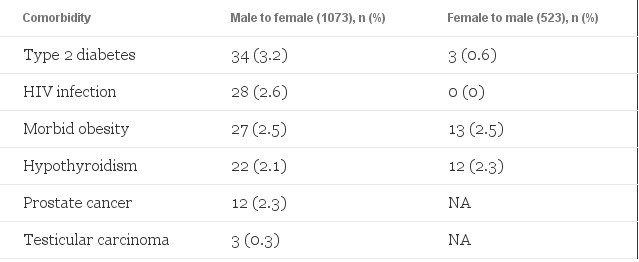
Unexpected Findings: Hypothyroidism and Male Cancers
An incidence of hypothyroidism greater than 2% in a population that was largely younger than 40 years is surprising, according to Dr. Asscheman, who said he would expect it to be closer to 1% in these younger adults.
However, 1 conference delegate said a 2% incidence of hypothyroidism in this sized population is likely.
Furthermore, session cochair Linda Nelson, MD, PhD, a reproductive endocrinologist from the University of Arizona College of Medicine, Phoenix, told Medscape Medical News thehypothyroidism rate may be unrelated to gender-dysphoria disorder or hormonal treatment.
Another unexpected comorbidity, according to Dr. Asscheman, was the occurrence of 7 male-specific cancers. He said this finding needs confirmation in other studies.
After cross-sex hormone treatment, side effects in MTF subjects, other than venous thromboembolism, included weight gain, reported in 5 persons (0.5%). In addition, 4 MTF subjects (0.4%) had a myocardial infarction, and 1 (0.1%) experienced a stroke. Hypertension was also common in this group, Dr. Asscheman stated.
The FTM subjects most often had the following side effects: acne with local treatment (2.9%, n = 15), weight gain (0.4%; n = 2), muscle pain (0.4%; n = 2), and liver-enzyme abnormalities (0.4%; n = 2).
One of Largest Database of Transgender Therapy; Results Reassuring
Dr. Asscheman noted several limitations of the retrospective study, including small numbers of patients with follow-up longer than 10 years or with an age above 60 years. In addition, some diagnostic rates differed between US and European centers.
A strength of the study, according to cochair Dr. Nelson, who was not involved with the research, was the size of the patient population. She called it "one of the biggest databases so far" of transgender patients receiving hormone therapy.
"Some side effects are expected, such as venous thromboembolism with estrogen use, but most of the results are really reassuring," she concluded.
Source - Joint Meeting of the International Society of Endocrinology and the Endocrine Society: ICE/ENDO 2014; June 24, 2014
Comments
comments powered by Disqus